Mechanical engineering graduate students Conor Walsh and Nevan Hanumara from the Massachusetts Institute of Technology (MIT) in Cambridge, MA, and graduate student Steven Barrett from the University of Cambridge in the U.K. have been working on the technology -- dubbed Robopsy -- since the fall semester of 2004.
The initiative began as a project in the precision machine design class of MIT professor Alexander Slocum. Five student teams were each given $4,000 to develop a specific product or technology in collaboration with a local doctor.
Dr. Rajiv Gupta, a radiologist at Massachusetts General Hospital (MGH) in Boston -- who also has an engineering background -- presented the soon-to-be Robopsy creators with the challenge of creating a small robot to help perform CT-guided precutaneous lung biopsies.
"Doctors come in (to the class) and present their ideas," Hanumara said in an interview with Auntminnie.com. "Out of that, I'd say three out of five ideas continue past the class."
|
|
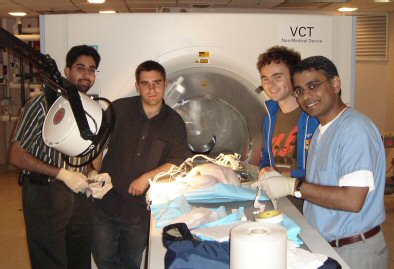
The Robopsy team (from left to right): Nevan Hanumara, Steven Barrett, Conor Walsh, and Dr. Rajiv Gupta. All images courtesy of the Massachusetts Institute of Technology.
|
Robopsy prototype
With conventional technology, doctors are not in the room when the CT scan is taken due to radiation exposure. After the patient image is acquired and reviewed, the physician returns to the room to find the suspicious mass manually.
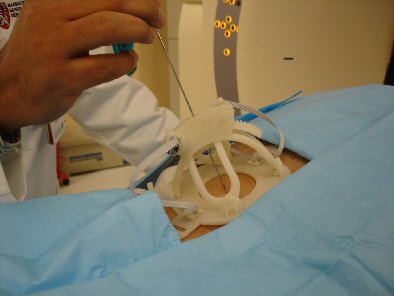
Robopsy is designed as a lightweight, dome-shaped plastic device that holds a biopsy needle and can rest on a patient's chest during a CT scan. With Robopsy, a doctor looking at the CT scan would use a laptop device to control the movement of the biopsy needle for increased precision and minimal intrusion.
|
|
Mock-up of the Robopsy device on a patient.
|
"If you don't need the robot to operate for a long period of time, and if you need reliability for just a single procedure, you can strip away much of the bulk and complexity," Hanumara said.
The advantages are that Robopsy would provide real-time needle position; rapid and slow incremental insertion and retraction; the ability for the doctor to "back drive" the mechanism instantly; and no shadows or "ghosting" in imaging.
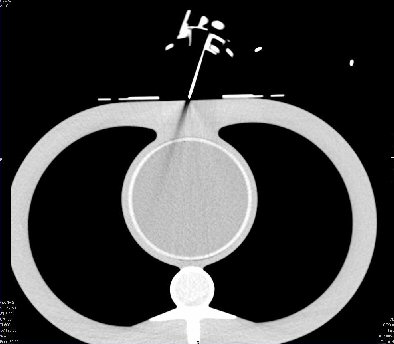
Validation trials were performed on a cardiac phantom at MGH using a 64-slice CT scanner from Siemens Medical Solutions of Malvern, PA. In a follow-up report, Gupta wrote that there was "negligible distortion to the CT image" from the hoop's actuators and that the biopsy needle "remained clearly visible."
|
|
CT image shows that Robopsy does not distort the image and creates no shadows that may obstruct the view of the needle's position.
|
For the patient's benefit, Hanumara estimates that Robopsy can reduce a two-hour biopsy procedure by approximately 20 minutes, shortening the time a patient is anesthetized and saving as much as $1,000 in per-procedure cost.
The major benefit is that the device would be more precise in the location and removal of suspicious masses. Initial testing also has shown that Robopsy can target lesions smaller than 5 mm in size. "That's another thing we hope to prove this fall with some animal trials," Walsh added.
The students plan to begin testing on pig cadavers, as MGH doctors develop a protocol to eventually test the device on a live pig. The live pig trials are expected to begin in September, with more testing to come over the next six months, as the device progresses toward U.S. Food and Drug Administration (FDA) 510(k) submission.
Commercial options
Hanumara and Walsh said their device is not limited to use for breast and lung biopsies, and may be used someday to investigate other applications in cancer biopsies. "If we can have a precise mechanical device between the doctor and the procedure, then we are taking advantage of the revolution provided by the imaging," particularly as it pertains to multislice CT, Walsh noted.
As for any commercial interest in Robopsy, Hanumara said the students remain "in the talking stage." They may opt for a financial partnership and "go it on our own," he said, "or do we want a production partner who will help us build and seek sales relations and production?"
While FDA clearance is still many months away, the students estimate that Robopsy will have a selling price in the range of $10,000. They also plan to market additional disposable actuator modules at an estimated cost of $500 each.
The trio already has received a fair share of notoriety by earning a $5,000 Boeing Prize at the 2005 MIT IDEAS Competition. In July, the invention finished in third place and received a cash prize in the Biomedical Engineering Innovation, Design and Entrepreneurship Award competition, sponsored by the National Collegiate Inventors and Innovators Alliance, based in Hadley, MA.
By Wayne Forrest
AuntMinnie.com staff writer
September 19, 2006
Related Reading
Dual-time-point PET spots local, distant breast metastases, September 14, 2006
Re-excision of margins reduces risk of local breast cancer recurrence after radiotherapy, August 30, 2006
Pulmonary nodule decisions must be individualized, August 16, 2006
Regular mammo screening reduces risk of false-positive results, needless biopsy
Management strategies evolve as CT finds more lung nodules, August 19, 2005
Copyright © 2006 AuntMinnie.com